Which carbon atom has an oxidation state of +3? 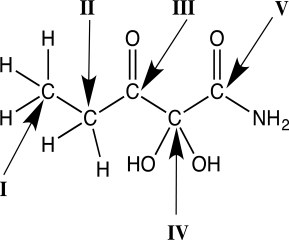
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q11: Evaluate the Lewis structure below and determine
Q12: Which orbital does not house core electrons
Q13: Which point on the following diagram represents
Q14: When two Lewis structures are related as
Q15: What is the molecular formula of this
Q17: Which electron configuration is correct for a
Q18: How many valence electrons are assigned to
Q19: Which of the following Lewis structures violates
Q20: How many total valence electrons are used
Q21: Which condensed formula contains an ester?
A)(CH3CH2)2O
B)CH3CO2CH2CH3
C)CH3CH2CH2COOH
D)CH3CH2OH
E)CH3COH
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents