Using a chemical reaction to convert an alcohol to an oxonium species is a highly valued tool in every organic chemist's arsenal (see the figure below).Although this reaction was not covered in the current chapter,it will be discussed in due course.Reconsider your response to the preceding question.Can you identify the two different atoms of the oxonium species to which a negatively charged species might be most attracted? Explain.Hint: It is not the oxygen with the positive charge. 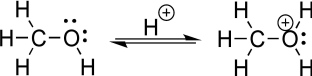
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q22: A compound with a molecular formula of
Q23: Which condensed formula contains a carboxylic acid?
A)CH3COCH3
B)CH3CO2CH2CH3
C)CH3CH2CH2COOH
D)CH3CH2OH
E)CH3COH
Q24: Compare Structure A with Structures B,C,and D.Is
Q25: Predict which carbon atom should have the
Q26: Which of the following
Q28: Consider two solvents that are commonly used
Q29: Which condensed formula contains a ketone?
A)CH3COCH3
B)CH3CO2CH2CH3
C)CH3CH2CH2COOH
D)CH3CH2OH
E)CH3COH
Q30: Which individual structures below could be contributing
Q31: Which would you expect to be a
Q32: Using line structures,deduce individual resonance contributors from
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents