When applying VSEPR theory to determine the geometry about a central atom,it is important to count the number of electron groups.Separately consider the two atoms highlighted with an arrow in the molecule shown below.How many electron groups must be considered for each of these central atoms? 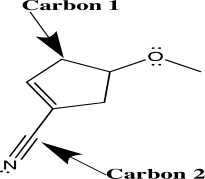
A) C1 has two groups; C2 has two groups.
B) C1 has three groups; C2 has four groups.
C) C1 has four groups; C2 has two groups.
D) C1 has four groups; C2 has three groups.
E) C1 has four groups; C2 has four groups.
Correct Answer:
Verified
Q4: Rotate the molecule below 180°,in the same
Q5: Turn the original molecule shown below 90°
Q6: What is the VSEPR geometry for the
Q7: Which of the following cycloalkanes contains a
Q8: Which of the following molecules contain(s)a nitrogen
Q10: The carbon atoms in the molecule below
Q11: Which cycloalkane contains a C-C-C bond angle
Q12: Which of the following statements is true
Q13: Which of the following molecules contains a
Q14: What are the approximate H-C-H bond angles
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents