When applying VSEPR theory to determine the geometry about a central atom,it is important to count the total number of bonded and nonbonded electron groups.Separately consider the two atoms highlighted with an arrow in the molecule shown below.How many bonded electron groups must be considered for each of these central atoms? 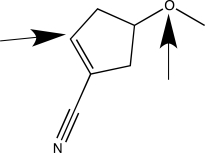
A) C has two groups; O has two groups.
B) C has three groups; O has four groups.
C) C has three groups; O has two groups.
D) C has three groups; O has three groups.
E) C has four groups; O has four groups.
Correct Answer:
Verified
Q32: Consider the structure of sodium benzoate,NaOC(O)Ph,the sodium
Q33: Which of the following functional groups contains
Q34: Select a phrase to complete this sentence:
Q35: Add substituents using dash-wedge notation to achieve
Q36: What is the strongest intermolecular attractive force
Q38: Rank the following molecules based on increasing
Q39: Which of the following intermolecular forces is
Q40: Identify the strongest intermolecular force.
A)Hydrogen bond
B)Ion-dipole
C)Ion-ion
D)Dipole-induced dipole
E)Induced
Q41: Rank N-N-dimethylaniline,phenethylamine,and phenethylamine hydrochloride in order of
Q42: Explain the chemical difference between a detergent
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents