How does the presence of the lone pair affect the geometry of the central atom in the following molecule? 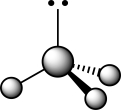
I The lone pair is attracted to the nuclei of the three substituents,creating larger bond angles.
II The lone pair repels the three sets of covalently bonded electrons.
III The lone pair has no bearing whatsoever on the VSEPR geometry at the central atom.
IVThe bond angles are smaller than a traditional tetrahedral bond angle due to lone pair repulsion.
A) I
B) II
C) III
D) IV
E) II and IV
Correct Answer:
Verified
Q17: Which cycloalkane has the greatest ring strain
Q18: Which cycloalkane contains a C-C-C bond angle
Q19: Which of the following choices correctly describes
Q20: What is the VSEPR geometry for any
Q21: Are any of the four 1,2-difluorocyclopropane isomers
Q23: Sodium chloride,an ionic compound,is highly water soluble
Q24: What is the strongest intermolecular attractive force
Q25: Identify the weakest intermolecular force.
A)Hydrogen bond
B)Ion-dipole
C)Ion-ion
D)Dipole-induced dipole
E)Induced
Q26: Rank the following molecules based on decreasing
Q27: A reverse micelle can form when a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents