How many hydrogen-bond donors and acceptors are present in the following molecule? 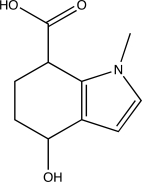
A) One donor and four acceptors
B) Two donors and four acceptors
C) Two donors and three acceptors
D) One donor and three acceptors
E) Two donors and two acceptors
Correct Answer:
Verified
Q23: Sodium chloride,an ionic compound,is highly water soluble
Q24: What is the strongest intermolecular attractive force
Q25: Identify the weakest intermolecular force.
A)Hydrogen bond
B)Ion-dipole
C)Ion-ion
D)Dipole-induced dipole
E)Induced
Q26: Rank the following molecules based on decreasing
Q27: A reverse micelle can form when a
Q29: Dimethyl sulfoxide (DMSO)is a polar aprotic solvent
Q30: When mixed,which of the following pairs of
Q31: Which functional group will engage in dipole-dipole
Q32: Consider the structure of sodium benzoate,NaOC(O)Ph,the sodium
Q33: Which of the following functional groups contains
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents