Naltrexone is an antagonist at the mu opioid receptor.What is the hybridization state and geometry of the nitrogen atom in naltrexone? 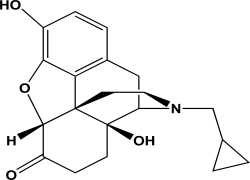
A) sp hybridized and linear geometry
B) sp2 hybridized and trigonal pyramidal
C) sp3 hybridized and trigonal pyramidal
D) sp3 hybridized and trigonal planar
E) sp3 hybridized and bent
Correct Answer:
Verified
Q7: Which two carbon atoms participate in the
Q8: Which of the following statements about molecular
Q9: Which characteristics describe the bonding MO for
Q10: According to valence bond theory,which atomic orbitals
Q11: Which two carbon atoms participate in the
Q13: Which of the following functional groups has
Q14: An atom's electrons have a high probability
Q15: Which of the following structures contains an
Q16: Which functional group has two sp2-hybridized oxygen
Q17: Boat A and Boat B start on
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents