Given below are 2s,2p,sp,sp2,and sp3 orbitals,all drawn to scale and in no particular order.Which of these orbitals would carbon use for a C-O π bond? 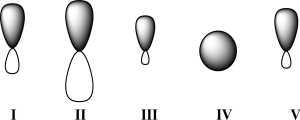
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q32: How many π bonds are present in
Q33: Given below are 2s,2p,sp,sp2,and sp3 orbitals,in random
Q34: For one or more of the following
Q35: Rank the carbon atoms in order of
Q36: For the given molecule,an amino alcohol,how many
Q38: Which two atomic orbitals overlap to form
Q39: What is the geometry and hybridization of
Q40: Which two atomic orbitals overlap to form
Q41: Bioymifi is a novel small molecule that
Q42: Amide bonds join amino acids together to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents