Styrene is an important industrial chemical in the generation of various plastics.Determine how many bonding molecular orbitals are created during the LCAO process to accommodate the π electrons in styrene. 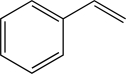
A) Two
B) Four
C) Six
D) Eight
E) Ten
Correct Answer:
Verified
Q26: Rank the carbon atoms in order of
Q27: For one or more of the following
Q28: Given below are 2s,2p,sp,sp2,and sp3 orbitals,all drawn
Q29: Given below are 2s,2p,sp,sp2,and sp3 orbitals,all drawn
Q30: Given below are 2s,2p,sp,sp2,and sp3 orbitals,all drawn
Q32: How many π bonds are present in
Q33: Given below are 2s,2p,sp,sp2,and sp3 orbitals,in random
Q34: For one or more of the following
Q35: Rank the carbon atoms in order of
Q36: For the given molecule,an amino alcohol,how many
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents