When the following molecule is treated with acid,it undergoes a reaction yielding a cyclic ether with the molecular formula C6H12O.Determine the structure of this product,and draw a mechanism to account for its formation. 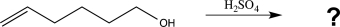
Correct Answer:
Verified
Q19: Predict the major product of the following
Q20: What is the most stable carbocation intermediate
Q21: Predict the major product of the following
Q22: Briefly explain why the hypothetical reaction below
Q23: Label the electron-rich and electron-poor species in
Q25: What would be the major product of
Q26: Draw a complete,detailed mechanism for the reaction
Q27: Draw a complete,detailed mechanism for the reaction
Q28: Which of the following,when treated with HBr,would
Q29: Give an example of a compound containing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents