Assume that the following molecule is planar.Use the Frost method to explain whether it is aromatic,antiaromatic,or nonaromatic.In your Frost diagram,fill in all π electrons,and label the HOMO and the LUMO. 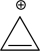
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q36: How many electrons are in the following
Q37: Is the following compound aromatic,antiaromatic,or nonaromatic? Explain
Q38: How many electrons are in the following
Q39: Which heterolysis step will occur fastest?
Q40: Is the following compound aromatic,antiaromatic,or nonaromatic? Explain
Q42: Is the following compound aromatic,antiaromatic,or nonaromatic? Explain
Q43: Is the following compound aromatic,antiaromatic,or nonaromatic? Explain
Q44: Is the following compound aromatic,antiaromatic,or nonaromatic? Explain
Q45: How many different π systems does the
Q46: For the following molecule,give the number of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents