Based on the spectrum below,calculate the approximate energy difference (in J)between the HOMO and LUMO of the molecule that created the spectrum. 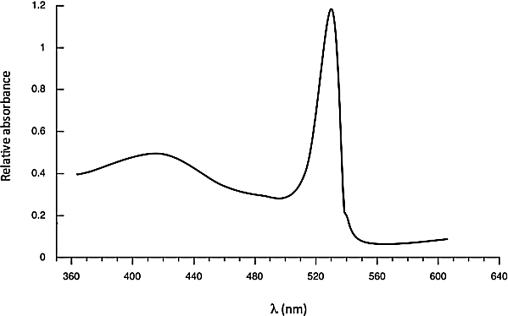
Correct Answer:
Verified
Q33: An unknown compound with the molecular formula
Q34: Which of the following compounds is consistent
Q35: How could you distinguish between the triple-bond
Q36: A sample has an absorbance of 0.68
Q37: Calculate the approximate wavelength (in nm)required to
Q39: Briefly explain why acetone absorbs at a
Q40: Which of the two molecules below would
Q41: An unknown compound with a molecular formula
Q42: A compound whose molecular formula is C5H8O
Q43: A compound has the molecular formula C7H6N2.Determine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents