What is true about the free energy diagram for the following reaction? 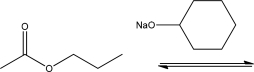
A) The first step of this reaction is the rate-determining step.
B) The free energy diagram for this reaction has three different transition states.
C) The overall products for this reaction include a carboxylic acid.
D) The first step of this reaction is exothermic.
E) The second step of this reaction is irreversible.
Correct Answer:
Verified
Q5: Which nucleophile will not readily react with
Q6: What is the most likely product of
Q7: Which of the following reagents will best
Q8: What is the most likely product for
Q9: Which of the following reagents can be
Q11: Which of the following reagents will best
Q12: What is the most likely product of
Q13: Which of the following reactions energetically favors
Q14: Which mechanism step is not required for
Q15: What is the most likely product of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents