Which of the following is true regarding the reaction shown below? 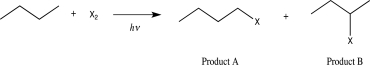
A) When X = Cl, the reaction will occur at a higher rate and will result in a lower ratio of Product A to Product B than when X = Br.
B) When X = Br, the reaction will occur at a higher rate and will result in a higher ratio of Product A to Product B than when X = Cl.
C) When X = Cl, the reaction will occur at a lower rate and will result in a lower ratio of Product A to Product B than when X = Br.
D) When X = Br, the reaction will occur at a lower rate and will result in a lower ratio of Product A to Product B than when X = Cl.
E) When X = Cl, the reaction will occur at a lower rate and will result in a higher ratio of Product A to Product B than when X = Br.
Correct Answer:
Verified
Q7: What type of elementary step is shown
Q8: Which of the following would result in
Q9: How many different radical species could be
Q10: Which of the following will undergo C-Cl
Q11: Which of the following elementary steps does
Q13: Which of the following steps is most
Q14: Predict the major products of homolytic C-C
Q15: Which of the following C-C bonds is
Q16: Which of the following is a termination
Q17: Predict the major product of the reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents