The chlorine radical abstracts a hydrogen atom from a secondary carbon in preference to a hydrogen atom on a primary carbon in a 4.5:1 ratio.Draw the two possible products that could be obtained from the reaction below,and compute the expected percentage of each product,taking into account the number of each type of hydrogen and the selectivity of chlorine. 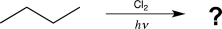
Correct Answer:
Verified
Q40: Draw single-barbed arrows to show homolytic bond
Q41: Design a synthetic route to accomplish the
Q42: Draw a complete,detailed mechanism for the reaction
Q43: Draw the propagation steps that occur in
Q44: Fill in the missing reactants needed to
Q45: Fill in the starting material and reagents
Q47: Devise a synthesis to carry out the
Q48: Predict the major product of the reaction
Q49: Predict the major product of the reaction
Q50: Arrange the compounds below in order of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents