What kind of molecular orbital (σ,σ*,π,or π*)results when the two atomic orbitals shown below interact in the manner indicated? 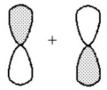
Correct Answer:
Verified
Q5: Consider the interaction of two hydrogen 1s
Q30: Draw the Lewis structure for CH3N2+.
Q31: What kind of molecular orbital (σ,σ*,π,or π*)results
Q32: What kind of molecular orbital (σ,σ*,π,or π*)results
Q33: What are the formal charges on nitrogen
Q34: The Kekulé structure of pentane is shown
Q36: Draw the Kekulé structure for each of
Q38: Give the formal charge on nitrogen in
Q39: Choose the correct hybridization for the atom
Q40: Draw a Lewis structure for the molecule
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents