Which carbon(s) in the following molecule is (are) sp hybridized? 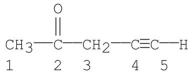
A) carbon 1
B) carbon 2
C) carbons 1,3
D) carbons 4
E) carbons 4,5
Correct Answer:
Verified
Q47: Among the hydrogen halides,the strongest bond is
Q48: The lone-pair electrons of the methyl anion
Q49: Determine the number of pi bonds in
Q50: The N-H bond in the ammonium ion,NH4+,is
Q51: What orbitals are used to form the
Q53: What orbitals overlap to create the H-C
Q54: A molecule of acetonitrile CH3CN contains _
Q55: The N-H single bond in methyl amine
Q56: How many sp2 hybridized carbons are present
Q57: What orbitals overlap to create the C-H
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents