Which of the following molecules has a net dipole moment of zero?
A) 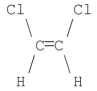
B) 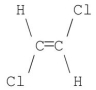
C) 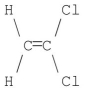
D) 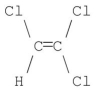
E) 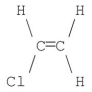
Correct Answer:
Verified
Q68: Why is the C-H bond in ethene
Q69: The carbon-carbon double bond in ethene is
Q70: Which of the following molecules has the
Q71: Give the hybridization,shape,and bond angle for the
Q72: Give the hybridization,shape,and bond angle for each
Q74: Identify the hybridization of the oxygen in
Q75: How many nonbonding electron pairs,bonding electron pairs,pi
Q76: Give the H-C-H bond angle in H2CO.
A)60
B)90
C)109.5
D)120
E)180
Q77: Draw the structure of a molecule which
Q78: Identify the compound with the weakest bond.
A)H2
B)HF
C)HCl
D)HBr
E)HI
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents