The lobes of the p-orbital are often designated by "+" and "-" signs as shown.What do these signs represent? 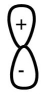
A) opposite charges
B) phases of the orbital
C) positive indicates the location of protons and negative indicates the location of electrons
D) that the orbital has polarity
E) all of the above
Correct Answer:
Verified
Q76: Give the H-C-H bond angle in H2CO.
A)60
B)90
C)109.5
D)120
E)180
Q77: Draw the structure of a molecule which
Q78: Identify the compound with the weakest bond.
A)H2
B)HF
C)HCl
D)HBr
E)HI
Q79: Which of the following molecules does not
Q80: Identify the hybridization of the nitrogen atom
Q82: Propanal is a compound detected on the
Q83: Several volatile compounds are responsible for the
Q84: Propanal is a compound detected on the
Q85: Several volatile compounds are responsible for the
Q86: Nitrous oxide N2O,also known as laughing gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents