What is the product formed from the following acid-base reaction when ammonia functions as a base? The equilibrium lies far to the reactants. 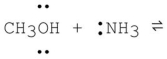
A) CH3O- + +NH4
B) CH2OH + +NH3
C) CH3OH2+ + -NH2
D) CH3NH2 + H2O
E) CH4 + NH2OH
Correct Answer:
Verified
Q13: Which species act as bases in the
Q14: The conjugate acid of H2O is
A)H3O-.
B)H3O.
C)H3O+.
D)HO-.
E)H2O+.
Q15: What is the conjugate acid of NH3?
A)(+NH3)
B)(-NH)
C)(+NH4)
D)(-NH2)
E)(+NH2)
Q16: What is the conjugate base of CH3NH2?
A)CH3NH3+
B)CH3NH-
C)NH4+
D)NH2-
Q17: Identify the compound with the highest pKa.
A)CH3NH2
B)CH3OH
C)CH3COOH
D)H2O
E)CH3NH3+
Q19: Identify the compound with the highest pKa.
A)CH3CH3
B)HCCH
C)CH2CH2
D)CH3OH
E)CH3NH2
Q20: Which of the following is the strongest
Q21: Identify the most acidic carboxylic acid.
A)ICH2COOH
B)BrCH2COOH
C)CH3COOH
D)FCH2COOH
E)ClCH2COOH
Q22: Predict the direction of equilibrium in the
Q23: Explain why AlCl3 is a Lewis acid.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents