What would be the conjugate acid in the following acid base reaction? 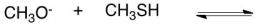
A) CH2O
B) CH3OH
C) CH3SH2+
D) CH3S-
E) H2O
Correct Answer:
Verified
Q33: Draw a resonance contributor and the resonance
Q34: At what pH will 25% of a
Q35: The pKa of CH3COOH is 4.8.If the
Q36: Which of the following is the strongest
Q37: Explain why : NF3 is a weaker
Q38: Which of the following anions,CH3CHBrCO2- or CH3CHFCO2-
Q39: At what pH will the concentration of
Q40: What would be the conjugate base in
Q41: What is the product of the following
Q43: Which of the following species cannot function
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents