Which of the following would have the highest boiling point?
A) CH3CH2-O-CH2CH2-O-CH3
B) CH3-O-CH2CH2CH2-O-CH3
C) HO-CH2CH2CH2CH2-OH
D) CH3CH2-O-CH2-O-CH2CH3
E) 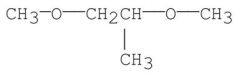
Correct Answer:
Verified
Q72: Explain why trimethylamine,(CH3)3N:,has a considerably lower boiling
Q73: Which of the following has the lowest
Q75: Which of the following has the greatest
Q76: What is the strongest intermolecular force present
Q79: Arrange the following amines in order of
Q80: Explain why the molecule shown below has
Q81: Draw the Newman projection of the most
Q82: Which of the following correctly lists the
Q85: Which of the molecules below has the
Q117: Which compound is more soluble in water?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents