Consider the reaction coordinate diagram shown.Which step has the greatest activation energy? 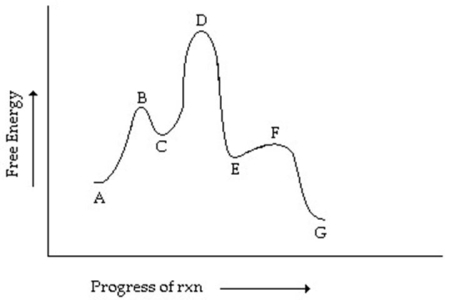
A) A going to C
B) C going to E
C) E going to G
D) E going to C
E) C going to A
Correct Answer:
Verified
Q68: Why do reactions tend to proceed at
Q69: Given an activation energy of 15 kcal/mol,use
Q70: How many degrees of unsaturation are present
Q71: The ΔG° for the conversion of "axial"
Q72: Which of the following correctly describes the
Q74: Consider the one-step conversion of F to
Q75: Based on the following energy diagram,which compound,A
Q76: Linalool is a major compound present in
Q77: The Arrhenius equation models how the rate
Q78: Consider the conversion of C to D
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents