Is the following molecule aromatic or antiaromatic? Explain. 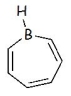
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q167: Which of the following is an isolated
Q168: What is the most acidic hydrogen on
Q169: Predict the product of the following reactions.
Q170: The C-C single bond in the following
Q171: The delocalized π system of benzene is
Q173: A dienophile with an electron withdrawing group
Q174: Predict the product of the following reactions.
Q175: Draw the resonance hybrid of the structures
Q176: Explain the reason for the difference in
Q177: There is large dipole moment associated with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents