How would you convert cyclopentadiene to the dialdehyde shown? 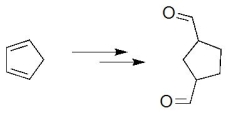
Correct Answer:
Verified
Q175: Draw the resonance hybrid of the structures
Q176: Explain the reason for the difference in
Q177: There is large dipole moment associated with
Q178: Draw the s-trans conformation of (2E,4Z)-4-methyl-2,4,-heptadiene.
Q179: Draw the s-cis conformation of (2E,4Z)-4-methyl-2,4,-heptadiene.
Q180: Which of the following statement is incorrect
Q181: Which of the following is the weakest
Q182: Show the missing reagents A and the
Q183: Which of the following is the strongest
Q185: Protonation of pyridine,shown below,generates a conjugate acid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents