Consider the following monobromination reaction,then answer the following questions. 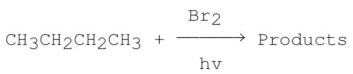 a)Give the structures and the IUPAC names for the products.
a)Give the structures and the IUPAC names for the products.
b)Give the common names for the products.
c)Calculate ΔH° for the overall reaction using the following data for the indicated bond dissociation energies:  d)Calculate the percent yield for each product.(relative rate of abstraction of 3° hydrogen is 1600; 2° is 82; and 1° is 1.)
d)Calculate the percent yield for each product.(relative rate of abstraction of 3° hydrogen is 1600; 2° is 82; and 1° is 1.)
e)Propose a step-by-step mechanism for the major product only.
f)Draw a schematic potential energy diagram for the rate-determining step (RDS)only.
g)Does the transition state of the RDS resemble more closely the reactants or the products?
h)Would the value of the activation energy be different for different alkanes? Explain.
i)Would the reaction slow down or speed up if I2 is used instead of Br2? Explain.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q3: Write an equation to describe the initiation
Q8: Chlorination of methane can result in a
Q29: When light is shown on a mixture
Q30: What is the major product of the
Q31: Which of the following most nearly describes
Q33: Identify as initiation,propagation,or termination. Q35: Consider the reaction: CH3CH2 ∙ + Br2 Q36: A hydrocarbon with molecular formula C5H12 is Q38: An alkane with the molecular formula C5H10 Q39: Which of the following is the most![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents