Calculate the percentage of 1-chloro-3-methylbutane in the following reaction. 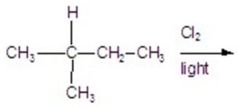
A) 11.56%
B) 27.77%
C) 23.14%
D) 35.18%
E) 13.88%
Correct Answer:
Verified
Q48: Calculate the percentage of 2-chloro-3-methylbutane in the
Q50: When butane undergoes free radical bromination,the product
Q51: An unknown sample is suspected of being
Q52: Calculate the percentage of 2-chloro-3,4-dimethylheptane formed in
Q55: List the following radicals in order of
Q56: Calculate the percentage of 3-chloro-3,4-dimethylheptane formed in
Q57: Calculate the percentage of 1-chloro-3,4-dimethylheptane formed in
Q58: Calculate the percentage of 2-chloro-2-methyl butane in
Q108: What is the relative reactivity of 2°
Q119: When 1,1,3,3-tetramethylcyclobutane is brominated at 125°C, the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents