Which of the following is an intermediate in the mechanism of hydrolysis of the ester shown below? 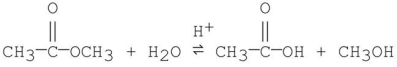
A) 
B) 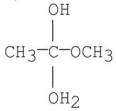
C) 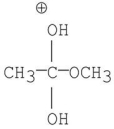
D) 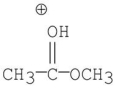
E) all of the above
Correct Answer:
Verified
Q10: How does a base catalyst increase the
Q11: Which of the following statements about how
Q12: Describe the acid-catalyzed second slow step in
Q13: Describe the acid-catalyzed first slow step in
Q14: How does an acid catalyst speed up
Q16: The reaction below occurs by a general-acid
Q17: Propose a step-by-step mechanism for the general-acid
Q18: An acid catalyst functions by donating _
Q19: Describe the reaction coordinate diagram for a
Q20: Describe the first step of a specific-base-catalyzed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents