What is the main purpose for using metal-ions as catalysts in the following hydrolysis reaction? 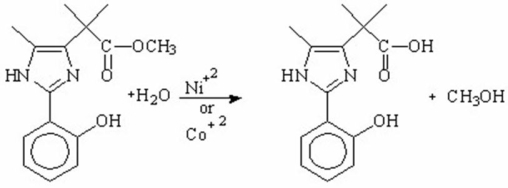
A) increases the rate by making water a stronger base,thereby increasing its electrophilicity
B) increases the rate by making water a stronger base,thereby increasing its nucleophilicity
C) increases the rate by making water a stronger acid,thereby increasing its nucleophilicity
D) increases the rate by making water a stronger acid,thereby increasing its electrophilicity
E) increases the rate by making water a stronger acid,thereby decreasing its nucleophilicity
Correct Answer:
Verified
Q30: Offer an explanation of how iodide catalyzes
Q31: Give the halide that is the most
Q32: How does a metal catalyst increase the
Q33: Show why typical metal-bound water has a
Q34: How does Cu2+ catalyze the decarboxylation of
Q36: Explain why an intramolecular reaction resulting in
Q37: Rank the following compounds in increasing order
Q38: Which of the following is a step
Q39: Describe the first step of a general-base-catalyzed
Q40: "Being in the right place...can be the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents