When 2,2-Dimethyloxirane (See Figure Below)undergoes Anionic Polymerization,the Nucleophile Attacks the Least
When 2,2-dimethyloxirane (see figure below)undergoes anionic polymerization,the nucleophile attacks the least substituted carbon of the epoxide,but when it undergoes cationic polymerization,the nucleophile attacks the most substituted carbon of the epoxide.Explain why this is so. 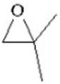
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q22: List the three phases in the mechanism
Q23: Which is not typically made through radical
Q24: In a recycling symbol,the _ the number
Q25: Propose a synthesis of poly(vinyl alcohol)from H2C
Q26: Which of the following is more likely
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents