Predict the products by completing a balanced equation for the following decomposition reaction.
CaCl2(l) 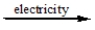
?
A) CaCl2(l) 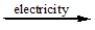
Ca(l) + 2Cl-(l)
B) CaCl2(l) 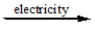
Ca2+(l) + Cl2(g)
C) CaCl2(l) 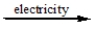
Ca2+(l) + 2Cl-(l)
D) CaCl2(l) 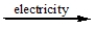
Ca(l) + Cl2(g)
E) CaCl2(l) 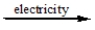
CaCl(l) + Cl-(l)
Correct Answer:
Verified
Q5: The combustion of an element is always
Q8: In an acid-base (neutralization) reaction the equivalence
Q12: All combustion reactions are classified as combination
Q14: A combination reaction may also be a
Q15: In an acid-base (neutralization) reaction the indicator
Q16: In a redox reaction, the oxidizing agent
Q77: Write down the oxidation number of the
Q79: In each of the following cases,write down
Q91: An aqueous solution of lead nitrate, Pb(NO3)2,
Q97: Complete and balance the equation for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents