Arrange the following gases in order of increasing rate of effusion.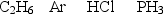
A) Ar < HCl <PH3 < C2H6
B) C2H6 < PH3 < HCl < Ar
C) Ar < PH3 < C2H6 < HCl
D) C2H6 < HCl < PH3 < Ar
E) Ar <PH3 < HCl < C2H6
Correct Answer:
Verified
Q49: At what temperature in Kelvin is the
Q50: Magnesium metal (0.100 mol) and a
Q53: Lithium oxide is an effective absorber
Q61: A compound composed of carbon, hydrogen, and
Q65: Which of the following gases will be
Q66: A gas mixture consists of equal masses
Q72: Hydrochloric acid is prepared by bubbling hydrogen
Q74: A 3.0-L sample of helium was placed
Q90: Select the gas with the largest root-mean-square
Q94: Select the gas with the highest average
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents