The highly exothermic thermite reaction,in which aluminum reduces iron(III) oxide to elemental iron,has been used by railroad repair crews to weld rails together.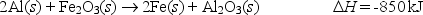
What mass of iron is formed when 725 kJ of heat are released?
A) 47 g
B) 65 g
C) 95 g
D) 112 g
E) 130 g
Correct Answer:
Verified
Q3: A system which undergoes an adiabatic
Q5: A system delivers 1275 J of
Q16: A system that does no work
Q18: A Snickers® candy bar contains 280
Q21: Which one of the following is
Q23: Benzene is a starting material in the
Q24: Calculate the enthalpy change for the
Q25: Which one of the following equations
Q26: Which one of the following is
Q27: Galena is the ore from which elemental
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents