Nitrogen and hydrogen combine to form ammonia in the Haber process.Calculate (in kJ) the standard enthalpy change H° for the reaction written below,using the bond energies given.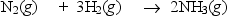
Bond:
N N
H-H
N-H
A) -969 kJ
B) -204 kJ
C) -105 kJ
D) 204 kJ
E) 595 kJ
Correct Answer:
Verified
Q6: Bond energy increases as bond order increases,
Q9: The majority of elements are good electrical
Q15: Covalently bonded substances do not necessarily exist
Q23: Which one of the following properties is
Q41: Based on electronegativity trends in the periodic
Q45: Which of the following elements is the
Q46: Covalent bonding typically occurs when a _
Q46: Select the most polar bond amongst the
Q53: Using appropriate, real examples to illustrate your
Q57: Which of the following elements is the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents