Hydrogenation of double and triple bonds is an important industrial process.Calculate (in kJ) the standard enthalpy change H° for the hydrogenation of ethyne (acetylene) to ethane.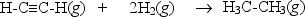
Bond:
C-C
C C
C-H
H-H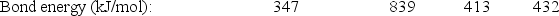
A) -296 kJ
B) -51 kJ
C) 51 kJ
D) 296 kJ
E) 381 kJ
Correct Answer:
Verified
Q24: Arrange oxygen,sulfur,calcium,rubidium and potassium in order of
Q30: Acetone can be easily converted to isopropyl
Q31: Arrange aluminum,nitrogen,phosphorus and indium in order of
Q41: Based on electronegativity trends in the periodic
Q47: Combustion of a fat will release more
Q48: Which of the following elements is the
Q50: When an atom is represented in a
Q52: Electronegativity is a measure of
A)the energy needed
Q55: In not more than three sentences, describe
Q56: When one mole of each of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents