Oxygen difluoride is an unstable molecule that reacts readily with water.Calculate the bond energy of the O-F bond using the standard enthalpy of reaction and the bond energy data provided.
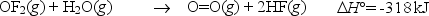

Correct Answer:
Verified
Q9: The majority of elements are good electrical
Q13: The lattice energy is the energy released
Q16: The more C-O and O-H bonds there
Q18: The lattice energy of large ions is
Q42: A hypothetical ionic substance will not form
Q51: Describe, with appropriate explanations, the key factors
Q52: Describe in brief how electronegativity values can
Q53: Using appropriate, real examples to illustrate your
Q56: Using appropriate, real examples to illustrate your
Q59: In not more than three sentences, describe
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents