Consider the phase diagram shown below. 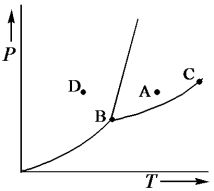
a.What phase(s)is/are present at point A?
b.What phase(s)is/are present at point B?
c.Name point C and explain its significance.
d.Starting at D,if the pressure is lowered while the temperature remains constant,describe what will happen.
Correct Answer:
Verified
b.solid,liquid and gas
c.C is t...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q18: The maximum number of phases of a
Q19: The energy of a hydrogen bond is
Q62: Mercury melts at -39°C and boils at
Q63: a. State the essential requirements for hydrogen
Q72: a.Explain what is meant by the
Q75: Chlorine trifluoride is used in processing nuclear
Q79: Liquid ammonia boils at -33.4°C and has
Q81: The highest temperature at which superconductivity has
Q82: A temperature increase causes _ in the
Q91: Use molecular orbital band diagrams to explain
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents