Procaine hydrochloride ( 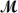
= 272.77 g/mol) is used as a local anesthetic.Calculate the molarity of a 4.666 m solution which has a density of 1.1066 g/mL.
A) 2.272 M
B) 4.056 M
C) 4.216 M
D) 4.666 M
E) None of these choices is correct.
Correct Answer:
Verified
Q34: The concentration of iodine in sea
Q40: Children under the age of six
Q41: Cinnamaldehyde ( Q42: Lysine is an amino acid that is Q45: Sodium hydroxide is a common ingredient in Q49: Aqueous ammonia is commercially available in a Q52: How many moles of solute particles are Q53: How many moles of bromide ions are Q78: Which of the following aqueous solutions should Q95: Human blood has a molar concentration of![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents