The Compound RX3 Decomposes According to the Equation
3RX3 \(\To\0 R + R2X3 + 3X2
In an Experiment
The compound RX3 decomposes according to the equation
3RX3 \(\to\0 R + R2X3 + 3X2
In an experiment the following data were collected for the decomposition at 100°C.What is the average rate of reaction over the entire experiment?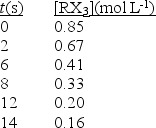
A) 0.011 mol L-1s-1
B) 0.019 mol L-1s-1
C) 0.044 mol L-1s-1
D) 0.049 mol L-1s-1
E) 0.069 mol L-1s-1
Correct Answer:
Verified
Q4: A study of the decomposition reaction
Q11: Consider the general reaction
5Br-(aq)+ BrO3-(aq)+ 6H+(aq)
Q12: Sulfuryl chloride, SO2Cl2(g), decomposes at high temperature
Q12: For the reaction
A(g)+ 2B(g)
Q13: Which one of the following sets of
Q14: Sucrose decomposes to fructose and glucose in
Q15: The rate constant for a reaction is
Q17: Which of the following sets of units
Q19: When the reaction A
Q20: Ammonium cyanate (NH4CNO) reacts to form urea
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents