In the gas phase at 500.°C,cyclopropane reacts to form propene in a first-order reaction.The figure below shows the concentration of cyclopropane plotted versus time.Use the graph to calculate approximate values of
a.the rate of the reaction,600.seconds after the start.
b.the half-life of the reaction,t1/2. 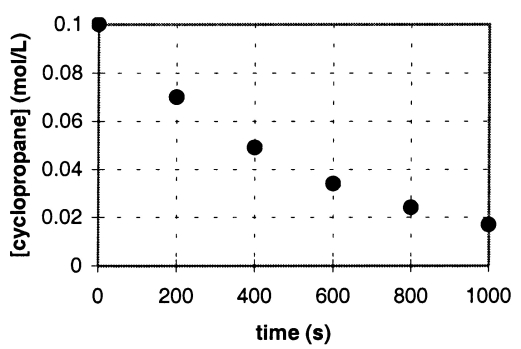
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q42: When a catalyst is added to a
Q43: A chemical reaction of the general
Q44: Which of the following affects the activation
Q50: At 25.0°C,a rate constant has the
Q51: In the gas phase at 500.°C,cyclopropane reacts
Q52: The gas-phase conversion of 1,3-butadiene to
Q59: You are studying the rate of
Q69: According to the collision theory of reaction
Q72: The elementary reaction HBr(g) + Br(g)
Q75: You are required to determine the energy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents