Cyclobutane decomposes to ethene in a first-order reaction.From measurements of the rate constant (k)at various absolute temperatures (T),the accompanying Arrhenius plot was obtained (ln k versus 1/T).
a.Calculate the energy of activation,Ea.
b.Determine the value of the rate constant at 740.K.(In the plot,the units of k are s-1. ) 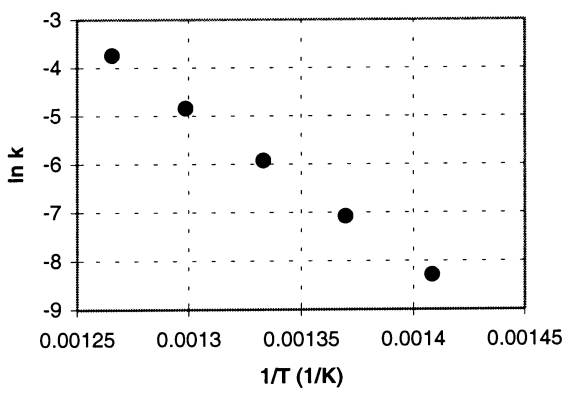
Correct Answer:
Verified
b....
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q36: A rate constant obeys the Arrhenius
Q37: The decomposition of dinitrogen pentaoxide has
Q38: The rate law for the reaction
Q39: Dinitrogen tetraoxide,N2O4,decomposes to nitrogen dioxide,NO2,in a
Q42: When a catalyst is added to a
Q43: A chemical reaction of the general
Q59: You are studying the rate of
Q61: The decomposition of dinitrogen pentaoxide to nitrogen
Q72: The elementary reaction HBr(g) + Br(g)
Q75: You are required to determine the energy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents