A B
At very low pressures many such reactions occur by the following mechanism:
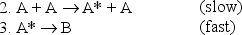
(A* represents a molecule with sufficient energy to overcome the activation energy barrier. )
a.Which of the three reactions above is/are elementary?
b.Where appropriate,identify the molecularity of the reactions.
c.Show that the proposed mechanism is consistent with reaction 1,the observed reaction.
d.Given the mechanism above,suggest a likely rate law for reaction (1).
Correct Answer:
Verified
b.Re...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: A transition state is a species (or
Q2: An elementary reaction is a simple, one-step
Q5: The half-life of a first-order reaction does
Q6: A reaction intermediate is a species corresponding
Q17: The rate of a reaction is determined
Q19: The units of the rate of reaction
Q61: Briefly list the features/properties common to all
Q63: The greater the energy of activation, Ea,
Q68: For each of the following terms/concepts, give
Q73: Is a bimolecular reaction necessarily second-order? Is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents