The equilibrium constant for the reaction of bromine with chlorine to form bromine monochloride is 58.0 at a certain temperature.
Br2(g) + Cl2(g) 
2BrCl(g)
What is the equilibrium constant for the following reaction?
BrCl(g) 
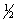
Br2(g) + 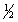
Cl2(g)
A) 2.97 10-4
B) 1.72 10-2
C) 3.45 10-2
D) 1.31 10-1
E) > 1.00
Correct Answer:
Verified
Q26: A mixture of 0.600 mol of
Q27: The equilibrium constant Kc for the reaction
A(g)+
Q28: Consider the equilibrium reaction: N2O4(g)
Q29: The equilibrium constant,Kp,has a value of
Q30: The equilibrium constant Kc for the reaction
PCl3(g)+
Q32: At 500°C the equilibrium constant,Kp,is 4.00
Q33: About half of the sodium carbonate
Q34: Nitrogen dioxide decomposes according to the
Q35: A mixture 0.500 mole of carbon monoxide
Q36: The reaction of nitrogen with oxygen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents