Consider the following two equilibria and their respective equilibrium constants:
(1) NO(g) + 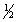
O2(g) 
NO2(g)
(2) 2NO2(g) 
2NO(g) + O2(g)
Which one of the following is the correct relationship between the equilibrium constants K1 and K2?
A) K2 = 2/K1
B) K2 = (1/K1) 2
C) K2 = -K1/2
D) K2 = 1/(2K1)
E) K2 = 1/(2K1) 2
Correct Answer:
Verified
Q33: About half of the sodium carbonate
Q34: Nitrogen dioxide decomposes according to the
Q35: A mixture 0.500 mole of carbon monoxide
Q36: The reaction of nitrogen with oxygen
Q37: The equilibrium constant,Kp,for the reaction
H2(g)+ I2(g)
Q39: Compounds A,B,and C react according to the
Q40: The equilibrium constant,Kc ,for the decomposition of
Q41: At 850°C,the equilibrium constant Kp for the
Q42: The reaction system Q43: Magnesium carbonate dissociates to magnesium oxide and
CS2(g)+ 4H2(g) ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents