The equilibrium constant for reaction (1) below is 276.Under the same conditions,what is the equilibrium constant of reaction (2) ?
(1) 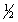
X2(g) + 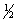
Y2(g) 
XY(g)
(2) 2XY(g) 
X2(g) + Y2(g)
A) 6.02 10-2
B) 7.25 10-3
C) 3.62 10-3
D) 1.31 10-5
E) None of these choices is correct.
Correct Answer:
Verified
Q17: Write the mass-action expression,Qc,for the following chemical
Q20: Write the mass-action expression,Qc,for the following chemical
Q22: Nitric oxide and bromine were allowed
Q23: Consider the equilibrium reaction: H2(g)+ Br2(g)
Q24: The two equilibrium constants for the same
Q24: Consider the reversible reaction: 2NO2(g) 
Q25: The equilibrium constant,Kp ,for the reaction
CO(g)+ H2O(g)
Q26: A mixture of 0.600 mol of
Q32: In order to write the correct mass-action
Q38: When a chemical system is at equilibrium,
A)the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents