A 20.0-mL sample of 0.30 M HClO was titrated with 0.30 M NaOH.The following data were collected during the titration.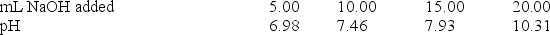
What is the Ka for HClO?
A) 1.1 10-7
B) 3.5 10-8
C) 1.2 10-8
D) 4.9 10-11
E) None of these choices is correct.
Correct Answer:
Verified
Q46: A 25.0-mL sample of 0.35 M HCOOH
Q52: A 25.0-mL sample of 0.10 M C2H3NH2
Q53: Write the ion product expression for calcium
Q54: A 50.0-mL sample of 0.50 M HCl
Q59: Write the ion product expression for magnesium
Q61: What volume of 0.500 M H2SO4 is
Q62: The solubility of lead(II) chloride is 0.45
Q64: A change in pH will significantly affect
Q66: The solubility of silver chromate is 0.0287
Q73: A 35.0-mL sample of 0.20 M LiOH
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents