Multiple Choice
Use the given data at 298 K to calculate G° for the reaction
2Cl2(g) + SO2(g) SOCl2(g) + Cl2O(g) 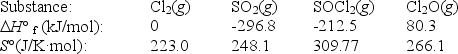
A) 129.3 kJ
B) 133.6 kJ
C) 196.0 kJ
D) 199.8 kJ
E) 229.6 kJ
Correct Answer:
Verified
Related Questions
Q45: Calculate the equilibrium constant at 25°C
Q46: What is the free energy change,
Q47: The reaction of methane with water to
Q48: Iron(III)oxide can be reduced by carbon
Q51: Calculate
Q52: Use the thermodynamic data at 298
Q53: Calculate
Q54: Hydrogen sulfide decomposes according to the
Q55: Calculate
Q67: a. Explain what is meant by a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents