Consider the Figure Below Which Shows G° for a Chemical Process Plotted Against Absolute Temperature
Consider the figure below which shows G° for a chemical process plotted against absolute temperature.Which one of the following is an incorrect conclusion,based on the information in the diagram? 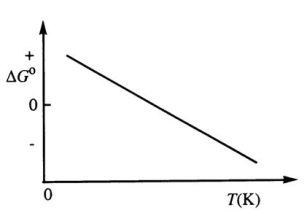
A) ( H° > 0)
B) ( S° > 0)
C) The reaction is spontaneous at high temperatures.
D) ( S° increases with temperature while H° remains constant.)
E) (There exists a certain temperature at which H° = T S°.)
Correct Answer:
Verified
Q38: Calculate
Q39: You are given pure samples of
Q41: Consider the figure below which shows
Q42: Sulfuryl dichloride is formed when sulfur
Q44: Nitric oxide reacts with chlorine to
Q45: Calculate the equilibrium constant at 25°C
Q46: What is the free energy change,
Q47: The reaction of methane with water to
Q48: Iron(III)oxide can be reduced by carbon
Q59: In order for a process to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents