Consider the Figure Below Which Shows G° for a Chemical Process Plotted Against Absolute Temperature
Consider the figure below which shows G° for a chemical process plotted against absolute temperature.From this plot,it is reasonable to conclude that: 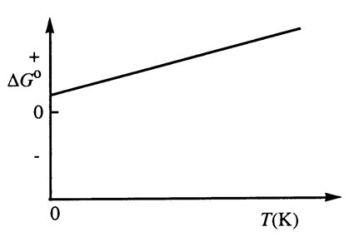
A) ( H° > 0, S° > 0)
B) ( H° > 0, S° < 0)
C) ( H° < 0, S° > 0)
D) ( H° < 0, S° < 0)
E) None of these choices is correct.
Correct Answer:
Verified
Q10: The higher the pressure of a gas
Q11: In some spontaneous processes, the entropy of
Q53: Calculate
Q54: Hydrogen sulfide decomposes according to the
Q55: Calculate
Q56: The temperature at which the following process
Q57: The temperature at which the following process
Q59: The formation constant for the reaction
Ag+(aq)+
Q60: Elemental boron can be formed by
Q61: Given: C2H2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents