A galvanic cell is constructed using the two hypothetical half-reactions 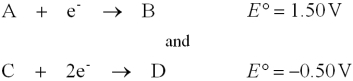
a.Write down the balanced equation representing the cell reaction.
b.Calculate the standard potential of this cell,E°cell.
c.Calculate G° for the cell reaction.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q5: Oxidation occurs at the cathode of a
Q11: In the shorthand notation for cells, a
Q73: The chlor-alkali process produces chlorine,Cl2(g),in large quantities.What
Q74: A concentration cell is based on
Q76: What product forms at the cathode during
Q79: Cryolite,Na3AlF6 is used in the electrolysis of
Q84: A solution is prepared by dissolving 32.0
Q90: Explain what is meant by a fuel
Q97: Which, if any, of the following metals
Q99: What mass of silver will be formed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents